Fda Emergency Use Authorization List Kn95
FDA Reverses Decision to Authorize Use of Chinese KN95 Respirators. However a N95 mask is NIOSH approved and for medical use.
Https Portal Ct Gov Media Dph Hai Covid19 Use Of Kn95s 05012020 Pdf
Recently after researching effectiveness the FDA removed its emergency use authorization for several manufacturers of KN95 masks.

Fda emergency use authorization list kn95. The Powecom KN95 face masks are on the FDAs Emergency Use Authorized list for medical settings and you can shop them on Amazon for under 2 each. They use elastic ear loops and feature an adjustable nose. On May 7 2020 FDA revised and reissued the Non-NIOSH-Approved Disposable Filtering Facepiece Respirators Manufactured.
For a full list of personal protective equipment authorized for emergency use by the FDA go to the agencys Emergency Use Authorizations webpage. On Thursday the FDA revoked that authorization for more than 65 of the 80 authorized manufacturers citing poor quality. The FDA guidance can be found here.
The FDA has approved several KN95 face masks on its Emergency Use Authorization list. Disposable KN95 Protective Mask Included on the FDA Emergency Use Authorization List Comfortable lightweight and effective this mask is your daily protection solution during travel work or leisure. According to Customs and Border Protection guidance the FDA does not object to importation and use of the KN95 respirators during the emergency.
Food and Drug Administration FDA has barred the importation of certain KN95 filtering facepiece respirators manufactured in China. Screenshot from Amazon FDA Emergency Use Authorization certified masks featuring multi-layer protection. N95 refers to the regulatory standard used in the US.
Health care workers in the fight against the COVID-19 pandemic. The JiaDaiFu KN95 masks are on the FDA emergency use authorization list and 100 percent satisfaction guaranteed by the seller on Amazon. Under an Emergency Use Authorization the FDA previously allowed the use of KN95s for US.
The remaining KN95 masks that demonstrated at least 95 filtration efficiency remain authorized for emergency use as respirators. Available in a 10-pack for 1999 from Amazon. KN95 refers to the regulatory standard used in China for filtering face respirators FFRs.
Powecoms KN95 face masks are on the FDA EUA List meaning the masksrespirators have been approved for Emergency Use Authorization in healthcare settings in accordance with CDC recommendations. To learn more about factors to consider when purchasing masks from another country including KN95 masks. So stick with best practicesand secure some Powecom KN95 masks for higher-risk settings.
We rounded up 6 FDA-approved KN95 masks that you can shop online at Amazon Vida and Maskc. This KN95 mask should be used in accordance with the most current CDC recommendations on optimizing respirator use. Is the FDA Emergency Authorized KN95 mask NIOSH approved.
Citing poor quality the US. KN95 masks that are approved with the FDAs emergency use authorization are actually hard to find these days. Powecom KN95 mask.
But not all brands of KN95 masks are created equal. Under a new emergency use authorization the FDA said that the KN95 devices which are similar to scarce N95 masks could be imported into the country and are eligible for authorization It stopped short of giving blanket approval for all of the KN95 respirators which are certified under Chinese rather than US health standards noting that it would only approve use of those that meet. According to Columbia University authentic KN95 respirators provide equivalent protection of an N95 respirator.
Powecoms KN95 face masks are on the FDA EUA List P meaning the masksrespirators have been approved for Emergency Use Authorization in healthcare settings. Youll be taking great care of yourself and others. On March 24 2021 the FDA revised this EUA to authorize for emergency use only those respirators listed in the EUAs Exhibit 1 as of the date of this reissuance.
 Fda Issues Enforcement Policies For Face Masks And Respirators Emergency Use Authorization For Ventilators Mcdermott Will Emery
Fda Issues Enforcement Policies For Face Masks And Respirators Emergency Use Authorization For Ventilators Mcdermott Will Emery
 Some Kn95 Masks Lose Fda Emergency Use Authorization Orthodontic Products
Some Kn95 Masks Lose Fda Emergency Use Authorization Orthodontic Products
 Amazon Com 11 Pack Kn95 Face Mask Headband Fda Eua List 95 Filtration Thick 4 Ply Disposable Face Masks For Adults Teens Women Men Medical Worker Clothing
Amazon Com 11 Pack Kn95 Face Mask Headband Fda Eua List 95 Filtration Thick 4 Ply Disposable Face Masks For Adults Teens Women Men Medical Worker Clothing

 Manufacturing And Distributing Respirators For Health Care Use In The United States Under An Existing Emergency Use Authorization Eua During The Covid 19 Pandemic Fda
Manufacturing And Distributing Respirators For Health Care Use In The United States Under An Existing Emergency Use Authorization Eua During The Covid 19 Pandemic Fda
Https Sonomawinegrape Org Wp Content Uploads Proforma Kn95 Masks Pdf
 Considerations For Selecting Respirators For Your Health Care Facility Fda
Considerations For Selecting Respirators For Your Health Care Facility Fda
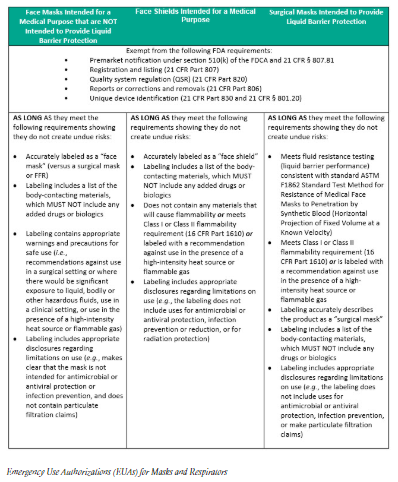
 Amazon Com Emercate Kn95 5 Layer Face Mask 20 Pcs Included On Fda Eua List Filtration 95 With Comfortable Elastic Ear Loop Non Woven Polypropylene Fabric Protection For Essential Workers Clothing
Amazon Com Emercate Kn95 5 Layer Face Mask 20 Pcs Included On Fda Eua List Filtration 95 With Comfortable Elastic Ear Loop Non Woven Polypropylene Fabric Protection For Essential Workers Clothing
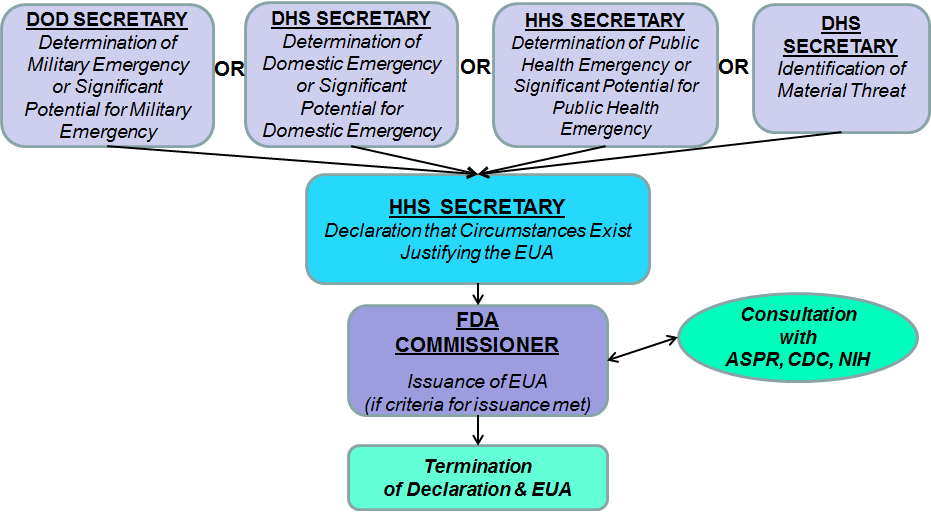
 Fda Updates Its Emergency Use Authorization For Kn95 Respirators From China
Fda Updates Its Emergency Use Authorization For Kn95 Respirators From China
Https Www Steris Com Media Documents Pdfs Covid19 Landing Page 6 9 Covid 19 N95 And N 95 Equivalent Respirator V Pro Decontamination External Qa Ashx
 Amazon Com 10 Pack Kn95 Face Mask Fda Eua List Ziplock Heat Sealed Pouch Earloops Thick 4 Ply Disposable Face Masks Particulate Respirator For Dust Haze Pm2 5 Adults Teens Healthcare Worker Use Industrial
Amazon Com 10 Pack Kn95 Face Mask Fda Eua List Ziplock Heat Sealed Pouch Earloops Thick 4 Ply Disposable Face Masks Particulate Respirator For Dust Haze Pm2 5 Adults Teens Healthcare Worker Use Industrial
 Fda Issues Enforcement Policies For Face Masks And Respirators Emergency Use Authorization For Ventilators Mcdermott Will Emery
Fda Issues Enforcement Policies For Face Masks And Respirators Emergency Use Authorization For Ventilators Mcdermott Will Emery
Comments
Post a Comment