Vaccine Testing Stages
Results of this first stage are expected next month and the expanded trial should have some reportable findings by June or July - although the study will run for a year. A number of other possible COVID-19 vaccines are in various stages of development and testing.
 13 Covid 19 Vaccines Are In Human Clinical Trials What Are They Technology Networks
13 Covid 19 Vaccines Are In Human Clinical Trials What Are They Technology Networks
Johnson Johnson launched the phase 3 trial for its experimental COVID-19 vaccine Sept.
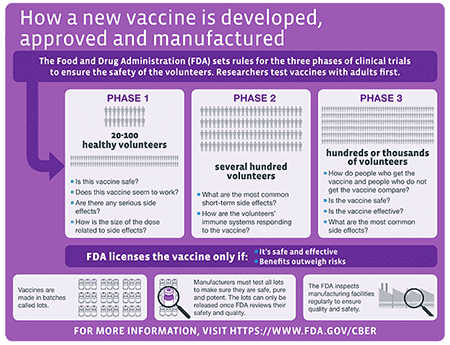
Vaccine testing stages. The scientists then conduct laboratory research to test their idea for a vaccine candidate. Standard vaccine development is a long process and studies are done in sequential steps. The fundamental scientific advances that make vaccine development possible arise from basic.
Its the only vaccine candidate made by a Canadian company for which the federal. These tests help confirm how the vaccines work and importantly to evaluate their safety and protective efficacy. Before any vaccine candidate can be released to the public it first has to go through several stages of study which researchers organize into discrete phases.
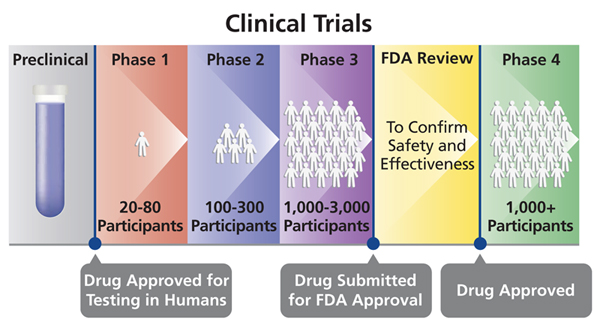
Laboratory testing and development. Phase 1 testing marks the first time the vaccine is tested in a small group of adults usually between 20 to 80 people to evaluate its safety and measure the immune response it generates. Quebec-based Medicago says its plant-based vaccine candidate made in partnership with GlaxoSmithKline GSK has reached the final stage of human testing.
These are some of the stages a vaccine will have gone through before use. Stages of Vaccine Development PRIORITY SETTING. On February 27 2021 the FDA granted EUA to an adenovirus vaccine developed by Johnson Johnson.
Coming up with a new idea or a variation on an existing idea. Wednesday November 18 2020 - 0659am. Scientists study their.
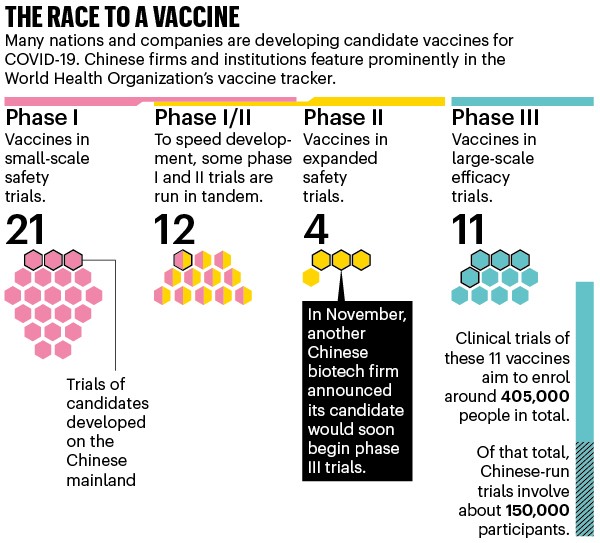
Approval of a vaccine requires completion of the following steps. The decision-making process for the development and production of vaccines should be guided by an. The state-owned China National Pharmaceutical Group Co Ltd Sinopharm is conducting Phase III clinical tests for two vaccine candidates developed by Wuhan Institute of Biological Products and Beijing Institute of Biological Products respectively with testing involving two dosages given 21 days apart.
TORONTO A COVID-19 vaccine created by a Canadian company is one step closer to becoming an option. Animals are infected with the virus. Chris Magee head of policy and media at UK non-profit Understanding Animal Research UAR told Full Fact that in the case of Covid-19 vaccines data already existed to indicate the vaccines were safe which enabled researchers to run animal trials alongside the early stages.
This is considered the Research and Discovery Stage. Theoretical development or innovation. Companies first make small batches and do small scale studies to characterise and optimise the production process.
Sometimes this testing occurs in animals. As we have written before the Covid-19 vaccines Pfizer Moderna and AstraZeneca have been tested on animals. 23 testing the single-shot candidates effectiveness in preventing COVID-19 against a placebo.
Primary efficacy analysis demonstrates BNT162b2 to be 95 effective against COVID-19 beginning 28 days after the first dose170 confirmed cases of COVID-19 were evaluated with 162 observed. This involves in vitro testing using individual. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate Meeting All Primary Efficacy Endpoints.
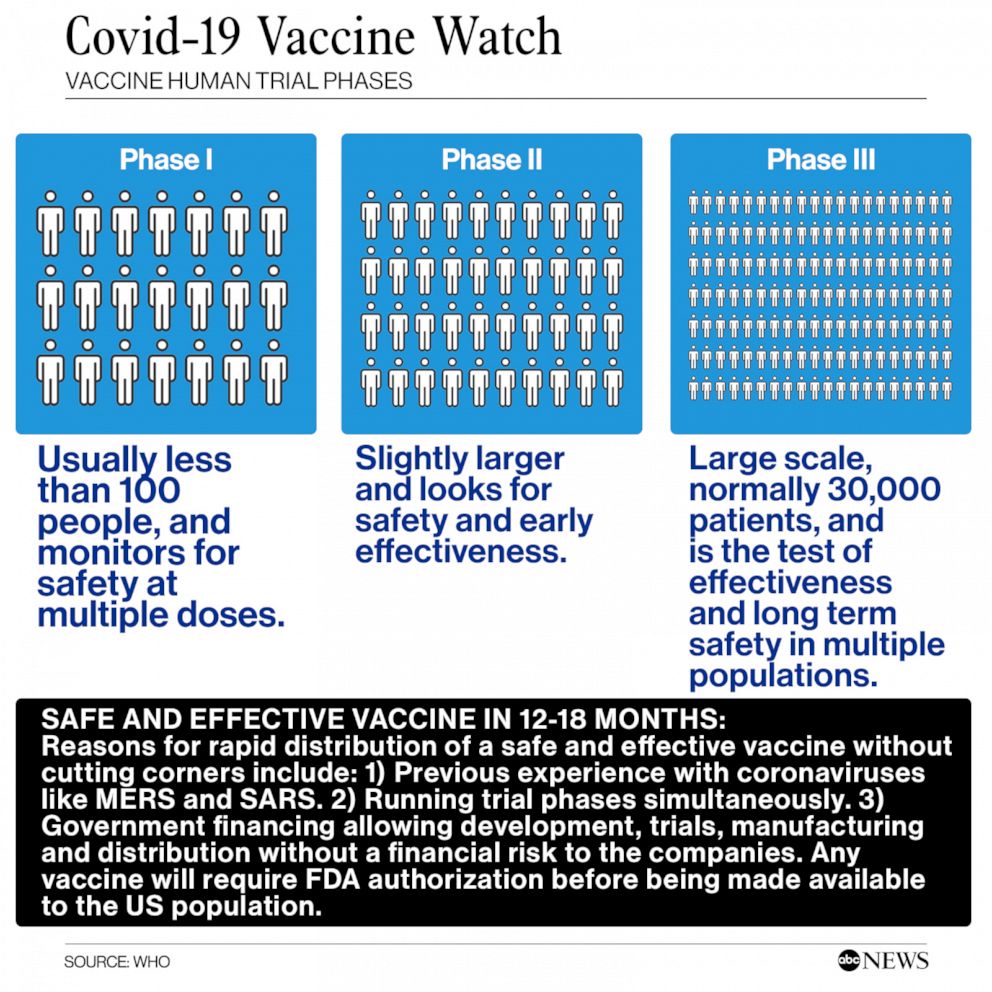
Phase 1 trials are relatively small trials usually around 100 volunteers with the primary objective of confirming the safety already strongly expected from animal studies. Phase 2a studies aim to determine the most effective dose and expand the safety experience with the vaccine. Reviewing what has been done before.
While these are the first mRNA vaccines to be rolled out to the general public the technology behind them has been developed over a number of years. CLAIM 2 - All were allowed to skip animal. A vaccine Phase I trial involves normal healthy subjects each tested with either the candidate vaccine or a control treatment typically a placebo or an adjuvant -containing cocktail or an established vaccine which might be intended to protect against a different pathogen.
BASIC AND APPLIED RESEARCH.
 Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News
Human Trial For Coronavirus Vaccine Launched By Moderna Enters Phase 3 Abc News
 How Soon Can We Have A Covid 19 Vaccine Times Of India
How Soon Can We Have A Covid 19 Vaccine Times Of India
 Inside China S Response To Covid
Inside China S Response To Covid
 First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
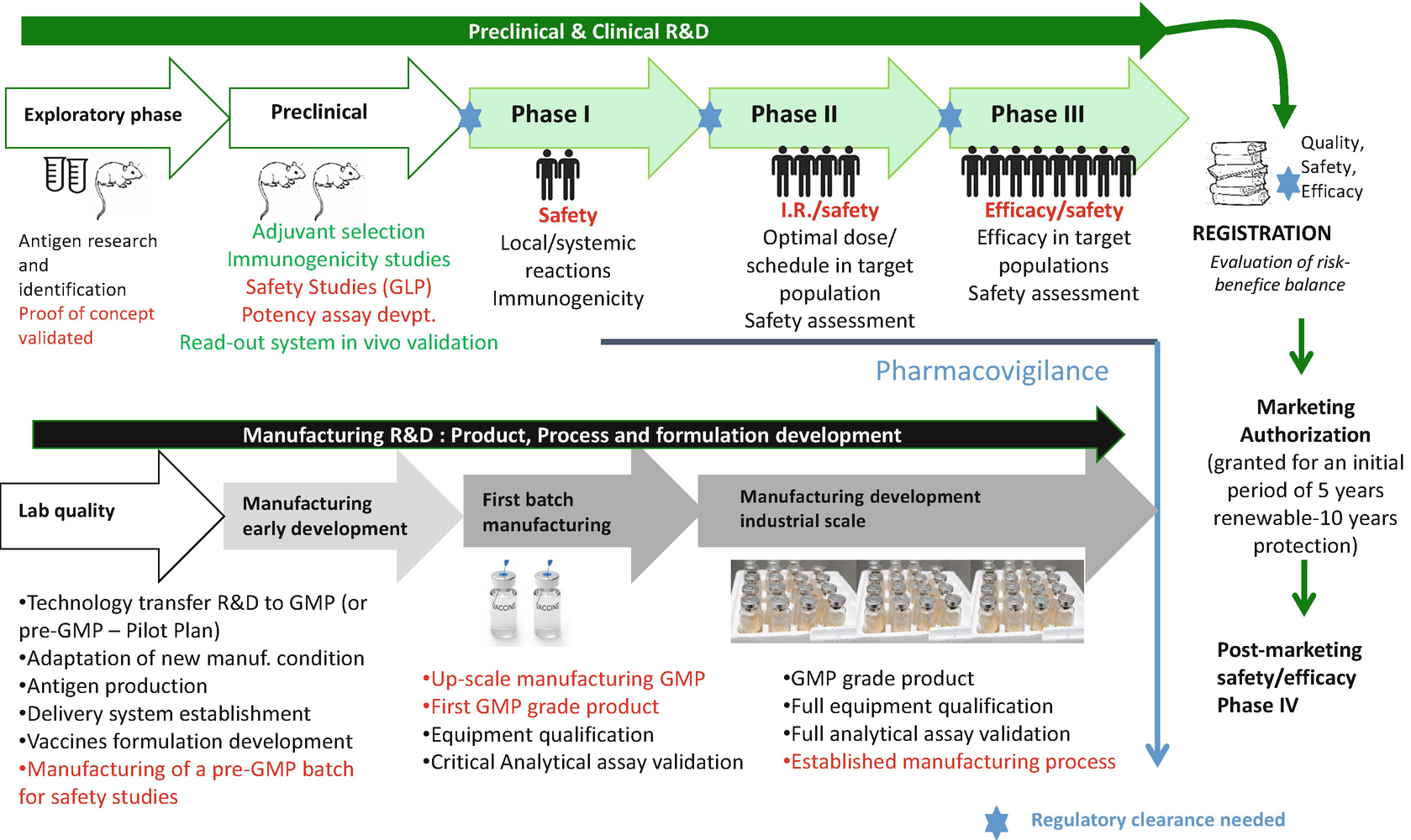 Vaccine Development From Preclinical Studies To Phase 1 2 Clinical Trials Springerlink
Vaccine Development From Preclinical Studies To Phase 1 2 Clinical Trials Springerlink
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
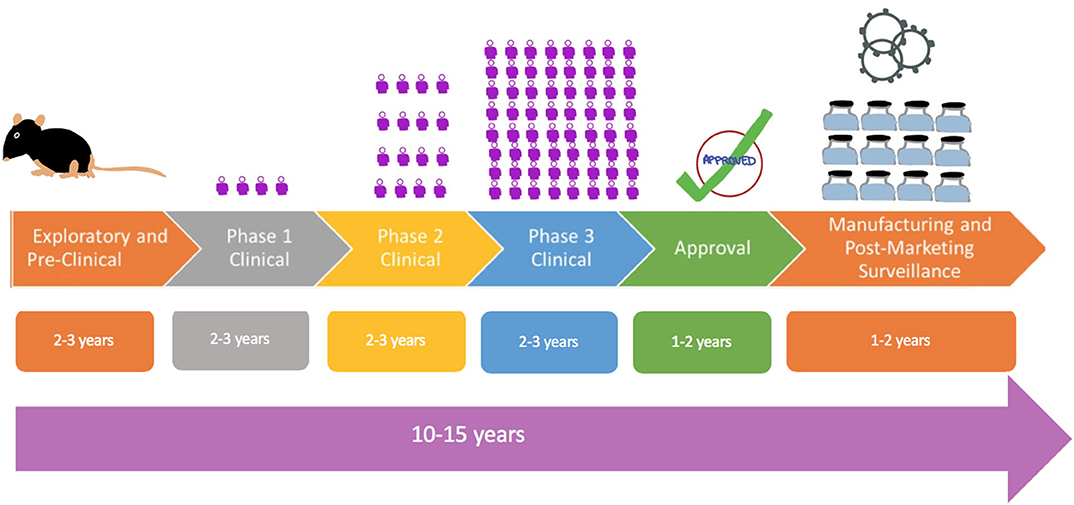 Frontiers A Review Of The Progress And Challenges Of Developing A Vaccine For Covid 19 Immunology
Frontiers A Review Of The Progress And Challenges Of Developing A Vaccine For Covid 19 Immunology
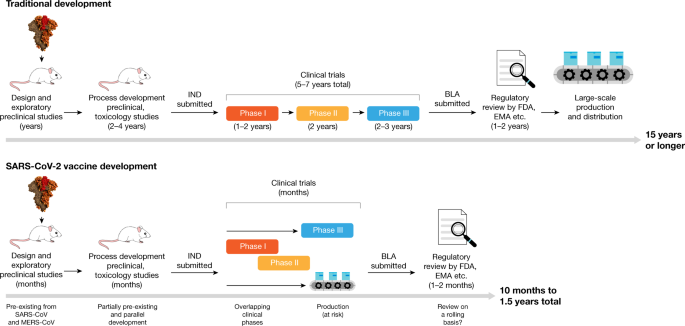 Sars Cov 2 Vaccines In Development Nature
Sars Cov 2 Vaccines In Development Nature
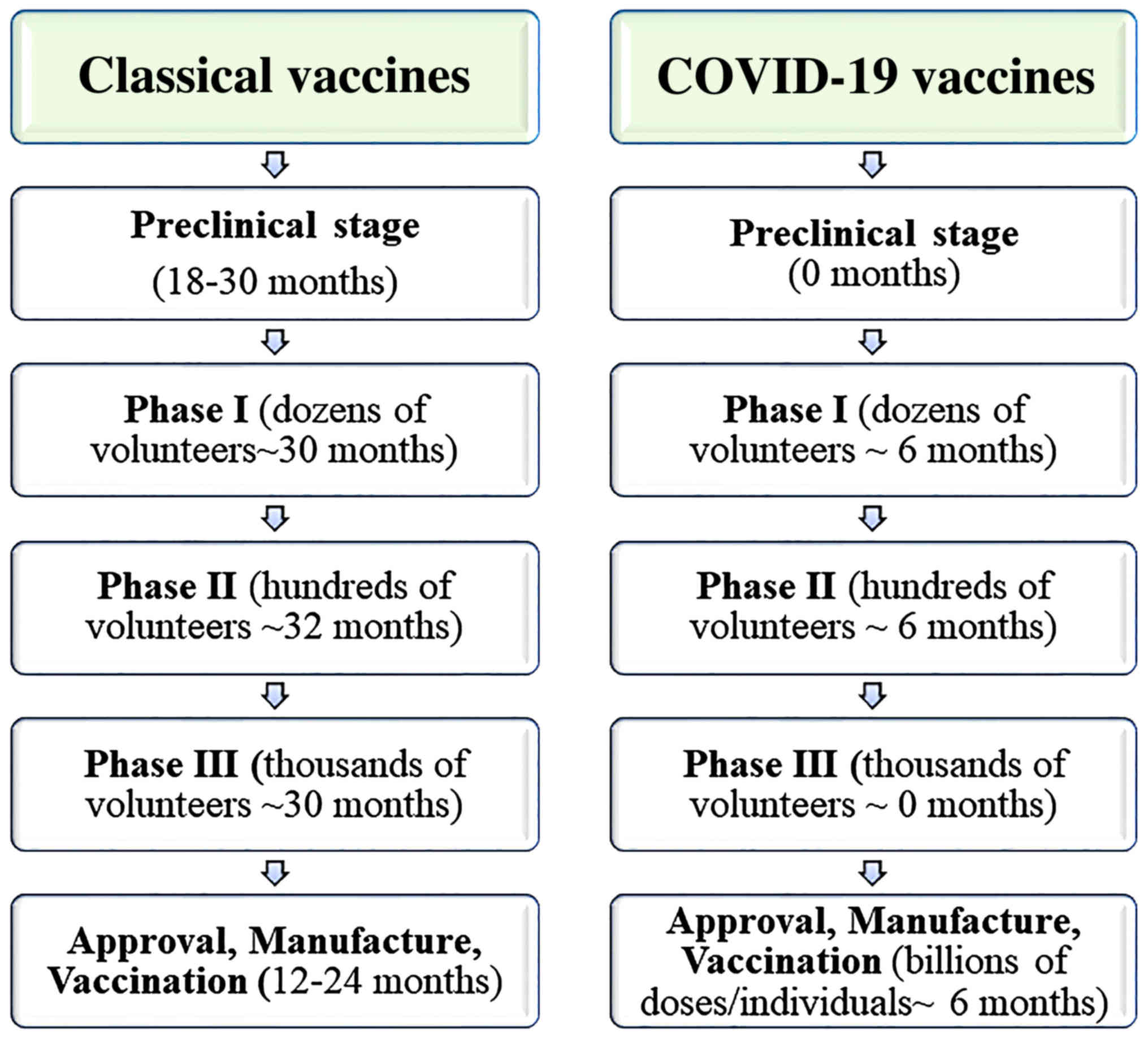 Towards Effective Covid 19 Vaccines Updates Perspectives And Challenges Review
Towards Effective Covid 19 Vaccines Updates Perspectives And Challenges Review
 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
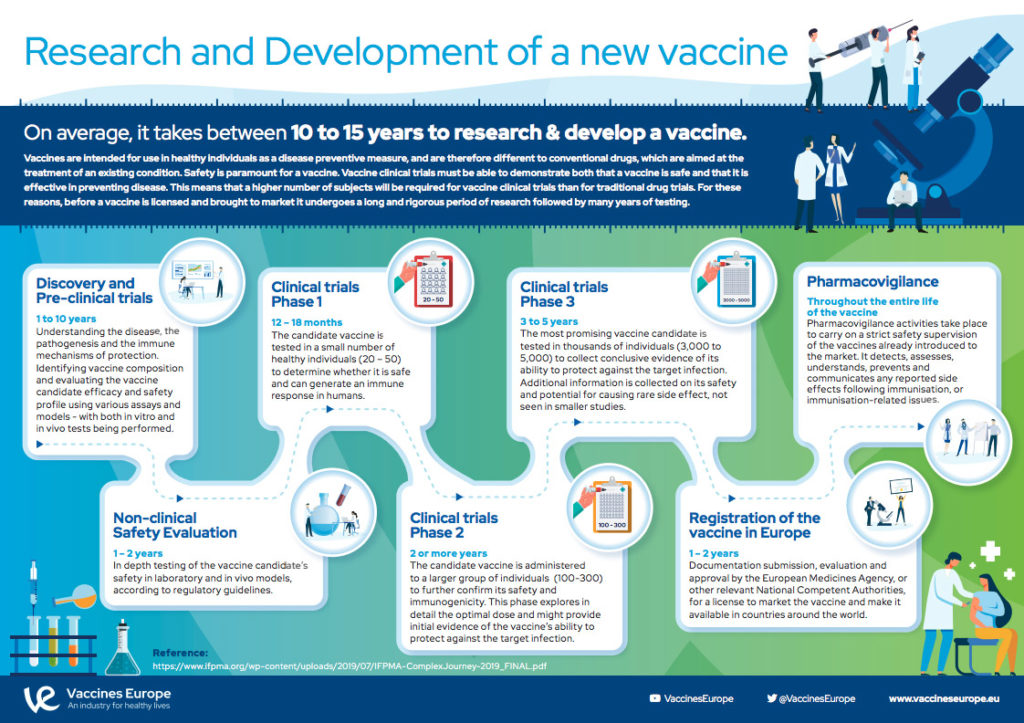 How Are Vaccines Developed Vaccines Europe
How Are Vaccines Developed Vaccines Europe
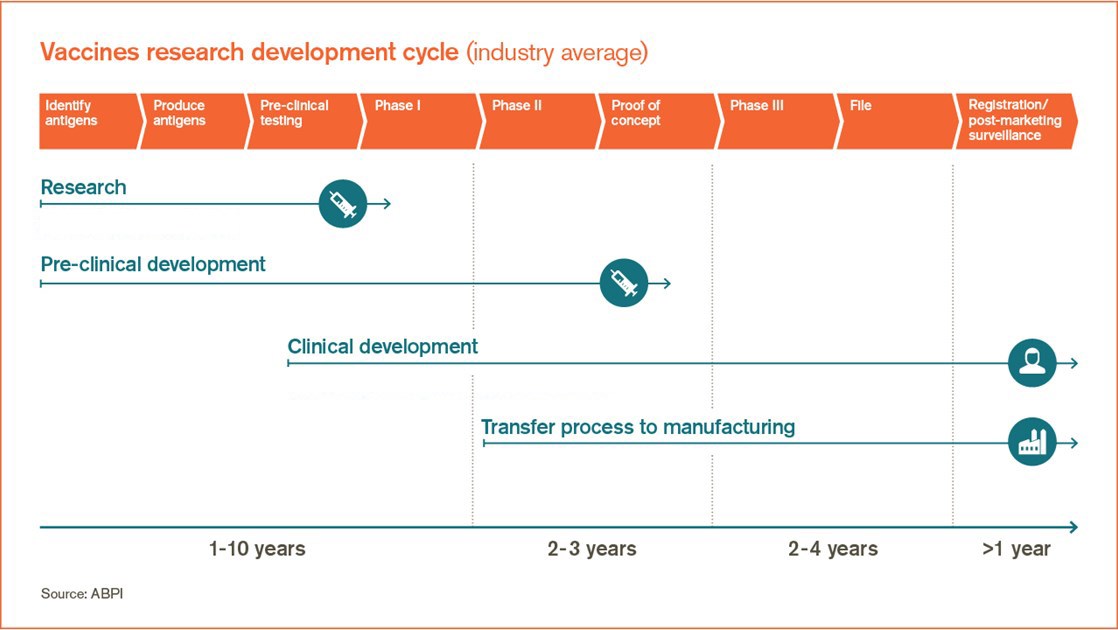 How We Develop New Vaccines Gsk
How We Develop New Vaccines Gsk
 Germany To Start First Coronavirus Vaccine Trial Germany News And In Depth Reporting From Berlin And Beyond Dw 22 04 2020
Germany To Start First Coronavirus Vaccine Trial Germany News And In Depth Reporting From Berlin And Beyond Dw 22 04 2020
Comments
Post a Comment