When Was Zantac Recalled
The FDA announced an official market withdrawal of Zantac on April 1 2020. 19 20190027 The recalled batches include certain lots of ranitidine tablets in 150 milligram and 300 mg strengths.
 What To Do During The Recall Of Prescription Zantac And Other Heartburn Medications Cbc News
What To Do During The Recall Of Prescription Zantac And Other Heartburn Medications Cbc News
Food and Drug Administration today announced it is requesting manufacturers withdraw all prescription and over-the-counter.

When was zantac recalled. If you took Zantac and developed cancer you may be able to recover compensation from the manufacturer. GET HELP 24 hours7 days a week 800 635-1144. April 02 2020.
Zantac was recalled by the FDA for containing NDMA a possible cause of cancer. On April 1 2020 the FDA requested manufacturers to withdraw all prescription and over-the-counter OTC ranitidine drugs Zantac others from the market immediately due to the presence of a contaminant known as N-Nitrosodimethylamine NDMA. 19 2020 537 PM PST.
Markets on April 1 2020. Apr 3 2020. 9 2019 when the online pharmacy Valisure told the FDA it found NDMA in samples of generic and brand name Zantac.
Appco Pharma has also. Only certain lots made by a single manufacturer were included in the recall but many retailers have pulled the product from their shelves completely as the FDA continues looking into the safety of the drug. What Zantac Has Been Recalled.
By the end of October many Zantac and ranitidine producers stopped distributing their products or recalled them. After the FDA conducted more testing the agency issued a request for all manufacturers to withdraw all ranitidine products from the market because of NDMA dangers. The events leading up to the Zantac recall started on Sept.
Home Practice Areas Zantac When Was Zantac Recalled. 23 Sandoz Inc a drug manufacturer that makes generic versions of Zantac announced that it was voluntarily recalling its ranitidine medicines because of confirmed contamination with N-Nitrosodimethylamine NDMA above levels established by the FDA in batches of Sandoz Ranitidine Hydrochloride Capsules. Food Drug Administration recalled Zantac from US.
The FDA announced the Zantac contamination on Sept. Zantac tablets are an oral over-the-counter product to prevent and relieve heartburn associated with acid. Recent recalls of popular antacids including Zantac and its generic version ranitidine as.
April 2020 Finally after months of testing the FDA released an official request for all Zantac and ranitidine products to stop being sold in the United States. The FDA asked manufacturers to. The FDA found significant contamination of cancer-causing toxins inside Zantac which increased the chance of cancer in the stomach liver and kidneys.
Health regulators are telling drugmakers to. Zantac and other heartburn drugs recalled over possible cancer link April 1 2020 424 PM AP Washington US. In addition to brand name Zantac generic Zantac has been recalled by Walgreens Walmart and.
The FDA also notified the public about a recall of nizatidine a chemically similar antacid. Call Pintas Mullins Law Firm to see what your legal options are. When was Zantac Recalled.
The private company said clues of the drugs potential risk can be traced to medical studies published since the early 1980s. This includes Zantac 150 Zantac 150 Cool Mint and Zantac 75. In September the FDA issued a recall for Zantac.
October 23 2019 -- As a precautionary measure Sanofi on Friday October 18 intiated a voluntary recall of all Zantac OTC over-the-counter in the United States. Zantac distribution halted due to contaminant Sept. In the months since the FDAs initial report and ranitidine recall multiple drug companies have announced additional recalls of the drug.
According to the FDA all ranitidine products including the oral liquidsyrup will be removed by their manufacturers and will not be available in the US. NDMA is the same carcinogen that led to a widespread recall beginning. The Food and Drug Administration has recalled the popular heartburn medication ranitidine known by the brand name Zantac.
Joshua Gagne PharmD ScD Contributor.
 Zantac Generics Ordered Off The Market After Fda Finds They Re A Ticking Time Bomb Fiercepharma
Zantac Generics Ordered Off The Market After Fda Finds They Re A Ticking Time Bomb Fiercepharma
 The Zantac Recall What To Do If You Take Ranitidine
The Zantac Recall What To Do If You Take Ranitidine
 Over The Counter Zantac Recalled In U S And Canada Supermarket News
Over The Counter Zantac Recalled In U S And Canada Supermarket News
 Zantac Recalled Because Of Possible Cancer Risk The Harrodsburg Herald
Zantac Recalled Because Of Possible Cancer Risk The Harrodsburg Herald
 Fda Demands Zantac Recall Napoli Shkolnik
Fda Demands Zantac Recall Napoli Shkolnik
 Zantac Ranitidine Voluntary Recall Information Gastroenterologist San Antonio
Zantac Ranitidine Voluntary Recall Information Gastroenterologist San Antonio
 Fda Requests Recall Of All Ranitidine Products On Us Market
Fda Requests Recall Of All Ranitidine Products On Us Market
Novartis And Glaxosmithkline Recall Versions Of Generic Zantac
 Zantac Heartburn Drug Recalled On Contamination Risk
Zantac Heartburn Drug Recalled On Contamination Risk
 Generic Zantac Is Recalled From Major U S Pharmacies After Carcinogen Is Found In Pills Los Angeles Times
Generic Zantac Is Recalled From Major U S Pharmacies After Carcinogen Is Found In Pills Los Angeles Times
 Fda Pulls All Forms Of Ranitidine From U S Market
Fda Pulls All Forms Of Ranitidine From U S Market

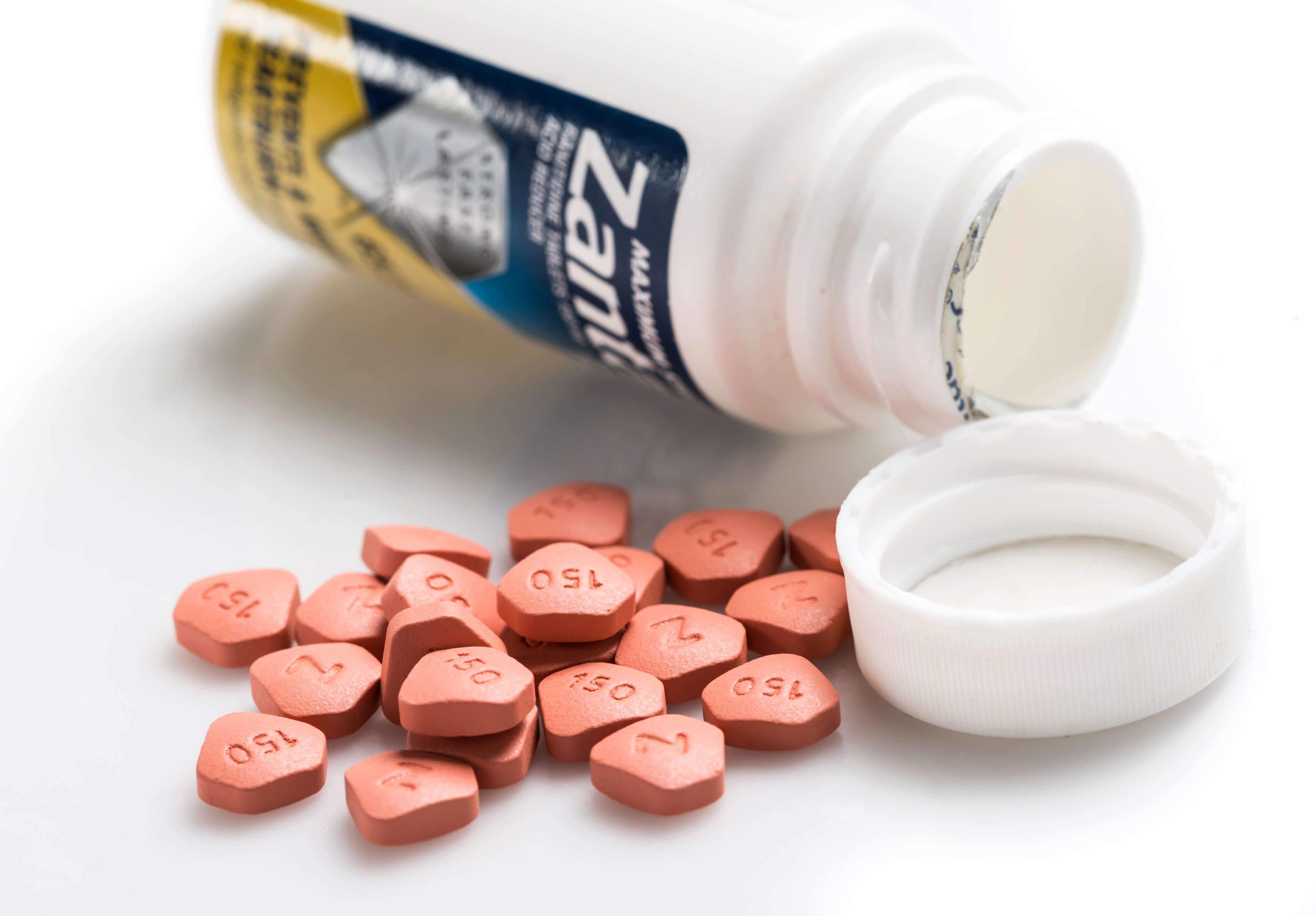 Fda Announces Generic Zantac Recalls Amid Cancer Concerns
Fda Announces Generic Zantac Recalls Amid Cancer Concerns
 Heartburn Drug Zantac Recalled Over Carcinogen Concerns
Heartburn Drug Zantac Recalled Over Carcinogen Concerns
Comments
Post a Comment